Efficacy
Clinical Studies
Several clinical studies have demonstrated the safety and efficacy of sacrosidase (Sucraid®) for the treatment of patients with Congenital Sucrase-Isomaltase Deficiency (CSID). Outlined below are a few of those studies and their findings.
In This Section
Study #1
In this study, Robayo-Torres and her colleagues examined both the ability of a 13C-labeled breath test to detect CSID without the need for a duodenal biopsy and the therapeutic effect of sacrosidase supplementation on breath test results. The patient population was 10 CSID patients, identified by low values in a biopsy sample sucrase activity assay and 10 control patients with dyspepsia or chronic diarrhea who had normal mucosal enzyme activity in the biopsy sample. Patients were separately administered uniformly labeled oral 13C-glucose and 13C-sucrose loads. After each administration, 13CO2 breath enrichment was assayed using an infrared spectrophotometer. Tests were repeated after sacrosidase administration. Results were quantified by calculating the mean percentage coefficient of glucose oxidation (%CGO).1
Results
Prior to treatment with sacrosidase, the average %CGO in the control group was 146% ± 45.5% as compared to only 25% ± 21% (P < .001) in CSID patients. These results demonstrate that the 13C-breath test was able to clearly distinguish between those patients with and those without CSID (Figure 1). Based on this outcome, the breath test demonstrated 100% sensitivity and 100% specificity (95% CI: 74%, 100% for both measures) in the detection of a low level of duodenal sucrase activity. All patients with CSID demonstrated a clinical response consistent with a correction of sucrase deficiency following oral sacrosidase supplementation (Figure 2; P = 0.001).1
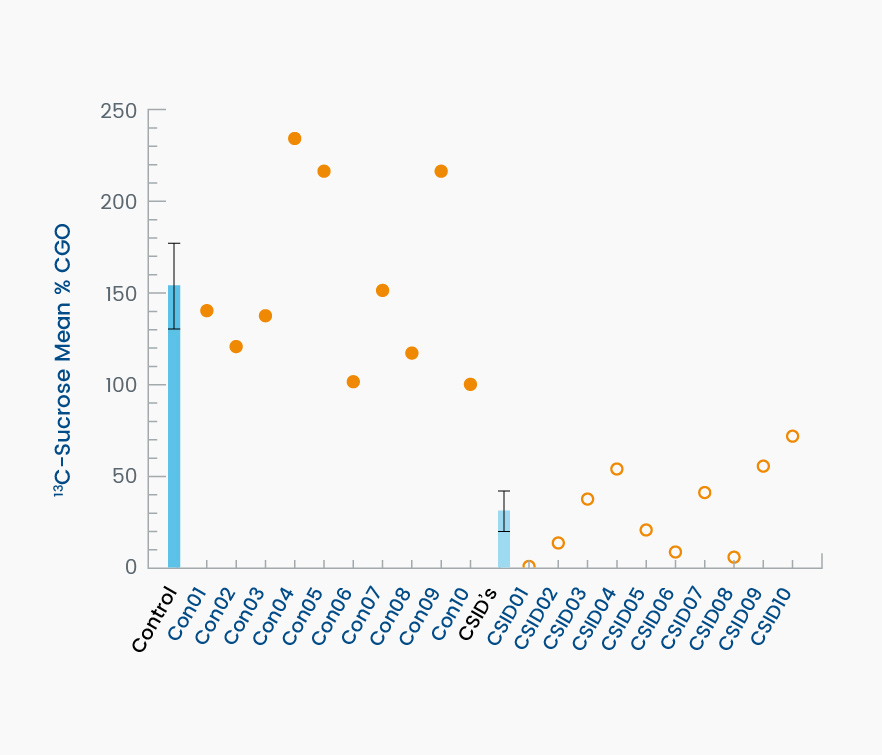
Figure 1. This graph demonstrates the ability of the 13C-labeled breath test to detect subjects affected by CSID. The darker blue bar on the left depicts the group average ± standard deviation (SD) of controls. Individual values are shown as filled circles. The lighter blue bar on the right depicts the average ± SD of the patients with CSID. Individual values are shown as open circles. The dashed line is the 79% mean %CGO reference value for discriminating between control and CSID subjects.1
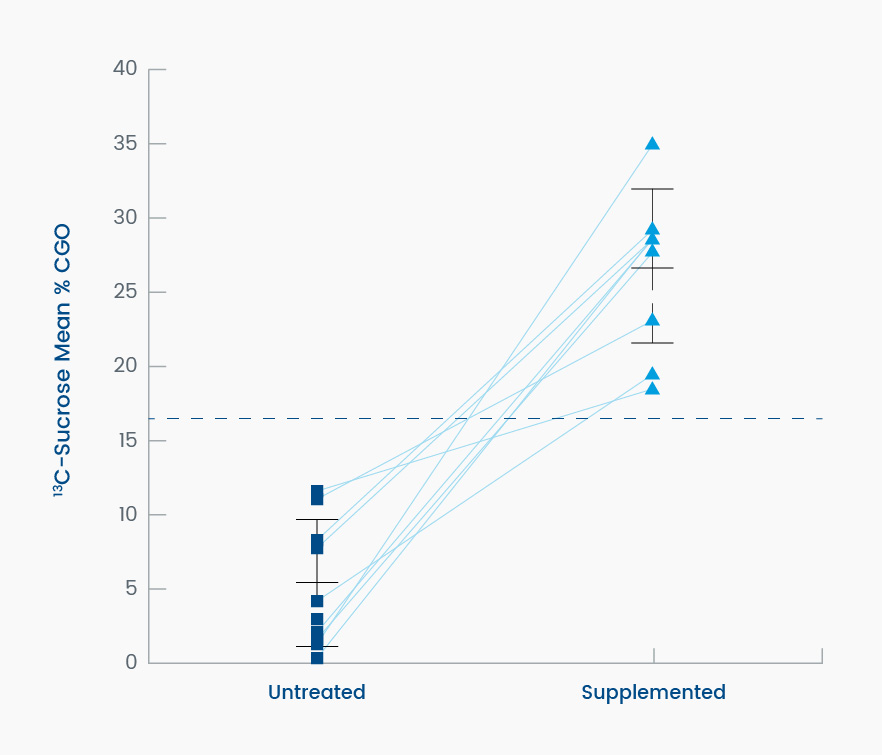
Figure 2. This graph dramatically demonstrates the ability of sacrosidase to metabolize radiolabeled sucrose in subjects with CSID who are sucrase deficient, measured as the change in mean percent coefficient of glucose oxidation (%CGO) on the sucrose breath test. The mean %CGO of individual patients with CSID untreated (left) and treated (right) with 22 drops of oral sacrosidase supplement added to the 20% solution sucrose load (P = .001; n = 9). The dashed line is the 79% mean %CGO reference value for discriminating between normal and untreated CSID subjects.1
Conclusions
The 13C-sucrose breath test was both sensitive and specific enough to confirm a CSID diagnosis in a noninvasive manner. In this study, all subjects with CSID demonstrated a clinical response consistent with a correction of sucrase deficiency following oral sacrosidase supplementation.1
Study #2
In a 1993 study conducted by Treem and his colleagues, the effects of yeast-derived sacrosidase on the hydrogen breath test response to an oral sucrose challenge was examined in a cohort of CSID patients. In addition, the ability of sacrosidase to reduce gastrointestinal (GI) symptoms of sucrose malabsorption was assessed.2
Study Design
Analytic methods were used to examine the enzymatic quality and stability of yeast-derived sacrosidase. The clinical activity of sacrosidase was evaluated in patients with a positive diagnosis of CSID who underwent double-blind, placebo-controlled hydrogen breath tests, followed by an eight-week, dose-response study conducted while consuming a diet containing moderate amounts of sucrose. During this study period, patients were treated with one of four different concentrations of yeast sacrosidase (YS), for 14 days at each dose.2
Results
Sacrosidase was found to be stable at 4˚C and retained full activity.
A total of 14 patients with CSID (five males and nine females; mean age 7.6 years) were enrolled in the study. All patients had normal lactase values and abnormal sucrase values on the hydrogen breath test. Overall, there was a significant reduction in cumulative hydrogen breath (P < .001) and peak hydrogen breath (P < .002) when sucrose was ingested with sacrosidase compared with placebo.2
Twelve patients completed the eight-week, dose-response phase of the trial and were administered one of four different concentrations of sacrosidase, ranging from a 1:100 dilution to a 1:100,000 dilution, during each 14-day period. Of note, there was a strong dose-response relationship; when these patients were administered the least diluted sacrosidase, they experienced the fewest gastrointestinal symptoms.2
Most patients tolerated a sucrose-containing diet with minimal symptoms while ingesting 1 mL of the 1:100-diluted sacrosidase with each meal. With increasingly dilute sacrosidase, symptoms of diarrhea, abdominal pain, and excessive gas were increasingly prevalent (Figure 3).2
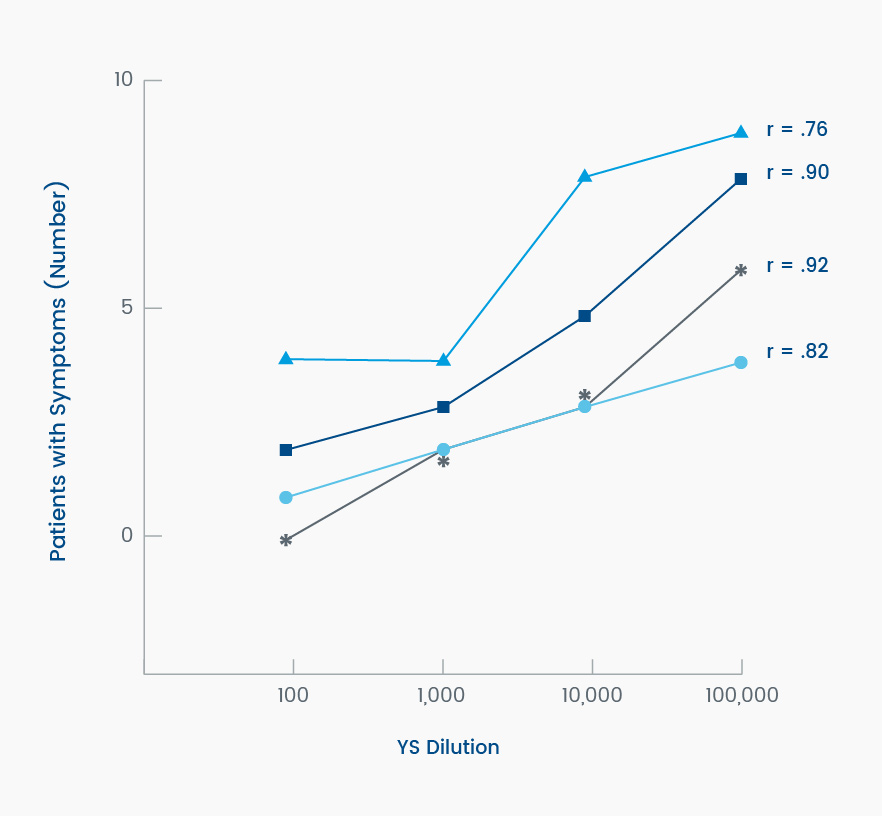
Figure 3. Symptomatic response of patients (N = 12) to increasing dilutions of yeast sacrosidase (YS) when consuming a sucrose-containing diet. Dilutions ranged from 1:100 to 1:100,000. Legend: *, >3 stools/d for ≥2 days of the 14-day period; [square], diarrhea on any day of the 14-day period; [circle], abdominal pain on any day of the 14-day period; [triangle], excessive gas on any day of the 14-day period. Adapted from Treem et al, 1993.2
Conclusions
In this preliminary study, sacrosidase was shown to be effective in reducing symptoms of CSID. The results indicated that early intervention in patients with CSID might prevent chronic, watery diarrhea and poor weight gain during infancy. There was a clear dose-response relationship, with the higher concentrations of sacrosidase providing greater efficacy. Sacrosidase remained stable at temperatures easily achievable in a domestic refrigerator.2
Study #3
In a study published in 1999, Treem and his colleagues examined the safety and efficacy of sacrosidase oral solution in children with confirmed CSID who were consuming a normal sucrose- and carbohydrate-containing diet.3
Patients Recruitment and Selection
Patients with CSID were recruited from the practices of members of the North American Society for Pediatric Gastroenterology.
Inclusion criteria were:2
- History of chronic, watery diarrhea with a pH <6.0
- Small intestinal biopsy specimens with measurements of tissue disaccharidase levels, where sucrase activity was <10% of control specimens
- Normal or decreased maltase activity
- Normal lactase levels and a normal result in a lactose breath hydrogen test
- Normal villous architecture of the small intestine
There were 28 patients with CSID enrolled in this randomized, multicenter, double-blind, controlled trial. All patients were infants or children (12 boys and 16 girls) between the ages of 5 months and 11.5 years (mean age, 4 years; median, 2.5 years). At baseline, the mean body weight of subjects was 16.5 kg.3
Study Design and Treatments
The trial consisted of two phases (Figure 4). The first phase consisted of a breath hydrogen test that included a challenge of 2 g/kg of a 20% sucrose solution plus one of three single-dose treatments: placebo, sacrosidase, or sacrosidase with milk. In the second phase, patients were randomized to one of four doses of sacrosidase for a 10-day treatment period and crossed over to other dosages in random order until a 40-day treatment period was completed. The administered volume of sacrosidase oral solution was weight-dependent: 1 mL/meal if body weight was ≤15 kg and 2 mL/meal if body weight was >15 kg.3
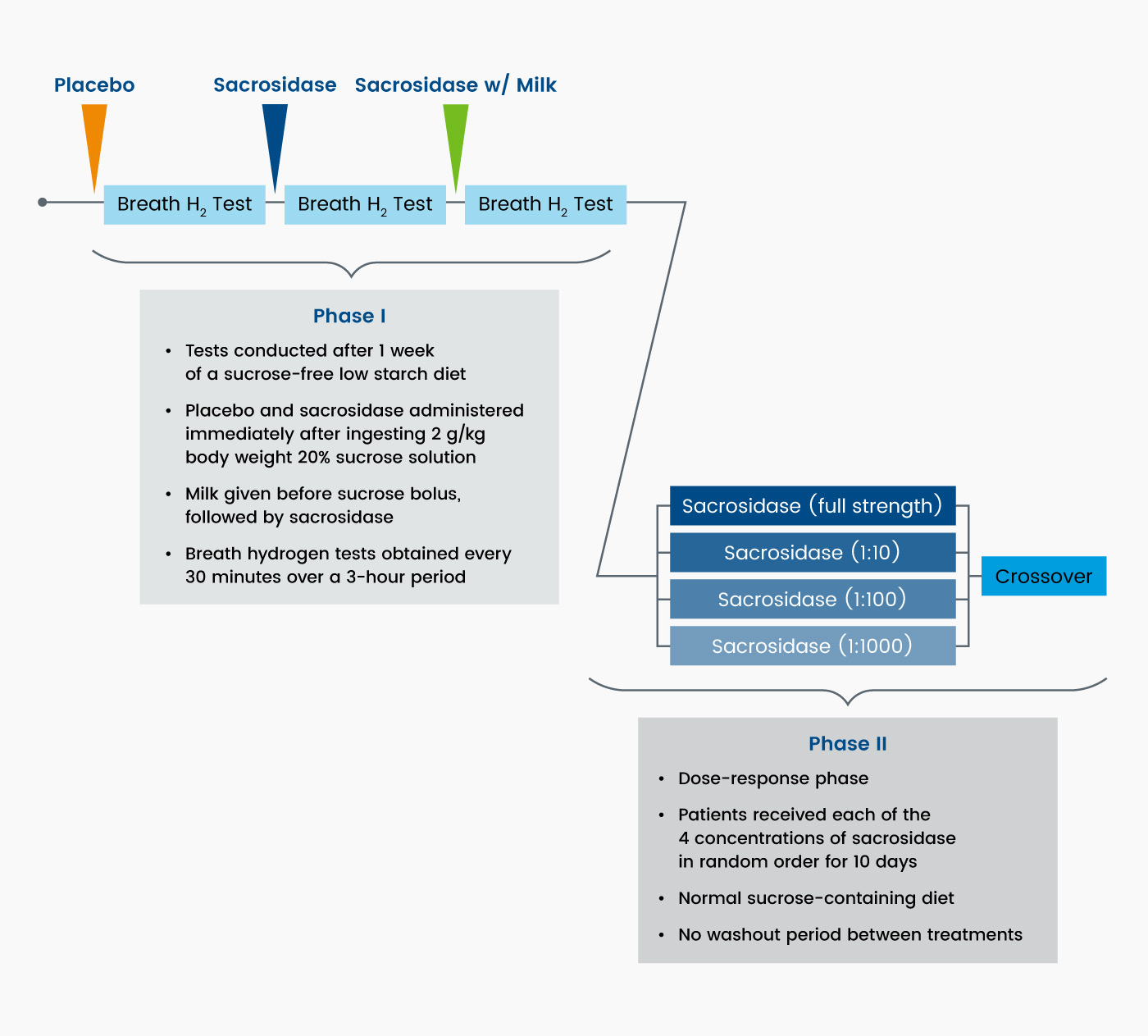
Figure 4. Crossover, dose-ranging study design3
Assessments and Endpoints
Stool frequency and consistency, symptoms, and dietary data were recorded daily and compared with baseline values obtained during the study period when the patients consumed a sucrose-free diet without sacrosidase. Dietary assessments of sucrose and carbohydrate consumption were summarized for each treatment period during the dose-response phase to verify whether patients were compliant with a normal diet, defined as 2 g/kg/d sucrose and 5 g/kg/d carbohydrate.3
The primary efficacy variables included the total number of stools and total symptom scores collected during the dose-response phase. All other measurements were secondary. During the dose-response phase, the number of stools and severity of symptoms (gas, bloating, nausea, vomiting, and abdominal cramps) were recorded daily by each patient and assigned values ranging from zero (none) to three (severe). A post hoc responder assessment (asymptomatic yes/no) was also determined.3
Results
Of the 28 patients in this trial who received ≥1 dose of sacrosidase, 26 (93%) completed both phases of the trial. All 28 patients participated in the first phase sucrase hydrogen breath test. When the subjects were administered the 20% sucrose solution plus placebo, the incidence of the subsequent gastrointestinal symptoms was: 81% diarrhea, 58% gas, 38% bloating, and 54% cramps.3
In the dose-response phase, significant differences were observed between the two higher concentrations (undiluted and 1:10 dilution) and the two lower concentrations of sacrosidase (1:100 and 1:1,000 dilution) for both primary outcome variables—total number of stools and total symptom scores Table 1).3
Table 1. Effect of sacrosidase on the number of stools and stool consistency during each 10-day period. Baseline values were obtained while consuming a sucrose-containing diet. Significant differences were shown between the two higher and two lower concentrations for the total number of stools (P ≤ .001) and total symptom scores (P = .003).
| Sacrosidase Treatment | Stools/d (Mean ± SEM) | Watery | Soft | Formed | Hard | Total Symptoms Score ≤7 (%) |
|---|---|---|---|---|---|---|
| Full strength | 1.82 ± 1.6 | 3.8 ± 1.7 | 6.1 ± 1.4 | 7.9 ± 1.2 | 0.4 ± 0.2 | 70 |
| 1:10 | 2.20 ± 2.5 | 7.0 ± 2.8 | 7.0 ± 1.2 | 7.7 ± 1.7 | 0.3 ± 0.2 | 63 |
| 1:100 | 2.58 ± 2.9 | 12.0 ± 3.3 | 8.3 ± 1.3 | 5.1 ± 1.1 | 0.1 ± 0.1 | 54 |
| 1:1000 | 2.44 ± 2.5 | 9.7 ± 2.3 | 9.0 ± 1.5 | 5.4 ± 1.1 | 0.3 ± 0.2 | 54 |
| Baseline | 2.31 ± 2.2 | 82 |
Overall, 81% of the patients were asymptomatic (defined as symptom-free for at least 7 of the 10 treatment days) while receiving full-strength sacrosidase; this incidence of symptom freedom and the gastrointestinal (GI) symptom scores were similar to the respective values recorded during the sucrose-free diet period.3 In patients up to 3 years of age, as many as 86% became asymptomatic, and in patients 3 years and older, 77% became asymptomatic. This difference suggests the therapeutic response to sacrosidase does not differ significantly by age (Figure 5).4
Only four patients in the trial experienced adverse events, including wheezing, vomiting, pallor, and dehydration. Three of these subjects completed the trial and continued in an open-label trial with sacrosidase. The remaining patient, who was hospitalized for wheezing and withdrew from the trial, had preexisting asthma. Most of the adverse events were attributed to concurrent illnesses common in childhood and judged probably unrelated to sacrosidase.3
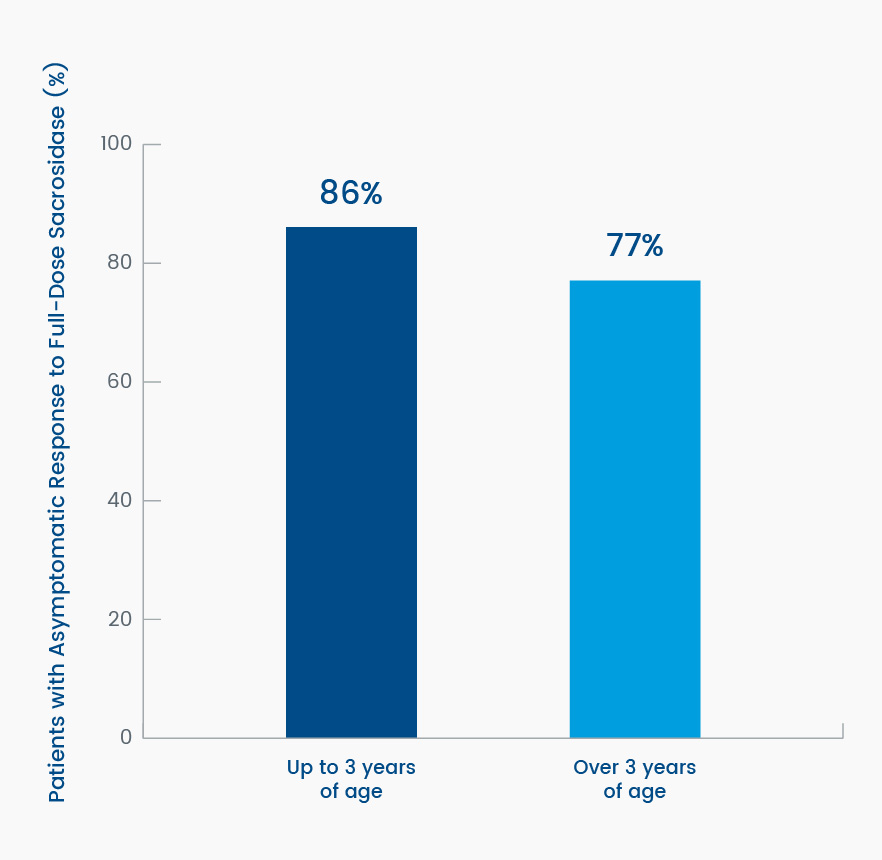
Figure 5. Differences in percent change from baseline in resolution of gastrointestinal symptoms (asymptomatic) to full-strength sacrosidase by subject age4
Conclusions
The results of this trial indicate that sacrosidase is an effective treatment for CSID. By taking sacrosidase with each meal, patients were able to eat a more normal carbohydrate- and sucrose-containing diet without developing major gastrointestinal symptoms. This study indicates that use of sacrosidase can reduce the high incidence of gastrointestinal complaints in this patient population.3
Study #4
This uncontrolled, open-label, long-term trial evaluated the safety, patient acceptability, and efficacy of sacrosidase in treating patients with CSID who were consuming a sucrose-containing diet. Upon completion of the previous controlled trials involving sacrosidase, 34 CSID patients from 6 months to 28 years of age (median age about 3 years) were allowed to continue treatment with sacrosidase for 2 to 54 months. Patient dosing varied from the currently approved dose.5
Results
Thirty-four CSID patients were treated in this trial, representing a total of over 900 patient-months of sacrosidase therapy. Initial management in this study was solely diet restriction. After institution of a sucrose-free and low-starch diet, the severity of gastrointestinal (GI) symptoms was reduced markedly. However, less than 10% of patients became asymptomatic and only 56% of patients were able to maintain a strict sucrose-free diet. In contrast, when placed on sacrosidase oral replacement therapy and a normal diet, 31 of 32 patients (97%) were rated overall as “improved significantly” by their parents. Forty percent of the patients were free of any symptoms, while the remaining 60% experienced only occasional, mild symptoms. Ratings of GI symptoms by the pediatric gastroenterologists and the primary care pediatricians were generally very similar to the responses given by the parents and patients, although the gastroenterologists tended to rate the symptom severity somewhat lower than the parents and pediatricians.5
A total of 31 adverse events were recorded in fourteen patients (41%). Some adverse events were considered as possibly related to treatment, while others were deemed unrelated. No patients discontinued treatment due to adverse events. Three patients experienced serious adverse events, and all three continued treatment with sacrosidase.5
Conclusions
The results of this long-term trial indicate that sacrosidase is well-tolerated and effective in the treatment of CSID as used in this trial. Sacrosidase allowed patients with CSID to consume a sucrose-containing diet over a long period, by attenuating the characteristic gastrointestinal (GI) symptoms typically experienced by these patients.5
References
- Robayo-Torres CC, Opekun AR, Quezada-Calvillo R, et al. 13C breath tests for sucrose digestion in congenital sucrase isomaltase-deficient and sacrosidase-supplemented patients. J Pediatr Gastroenterol Nutr. 2009;48(4):412-418. doi:10.1097/mpg.0b013e318180cd09
- Treem WR, Ahsan N, Sullivan B, et al. Evaluation of the liquid yeast-derived sucrase enzyme replacement in patients with sucrase-isomaltase deficiency. Gastroenterology. 1993;105(4):1061-1068. doi:10.1016/0016-5085(93)90950-h
- Treem WR, McAdams L, Kastoff G, Justinich C, Hyams J. Sacrosidase therapy for Congenital Sucrase-Isomaltase Deficiency. J Pediatr Gastroenterol Nutr. 1999;28(2):137-142. doi:10.1097/00005176-199902000-00008
- QOL Medical. LLC. Data on file, 1997.
- QOL Medical. LLC. Open-label, long-term study of sucrase enzyme therapy for Congenital Sucrase-Isomaltase Deficiency. OMC-SUC-3. Data on file, 1997.